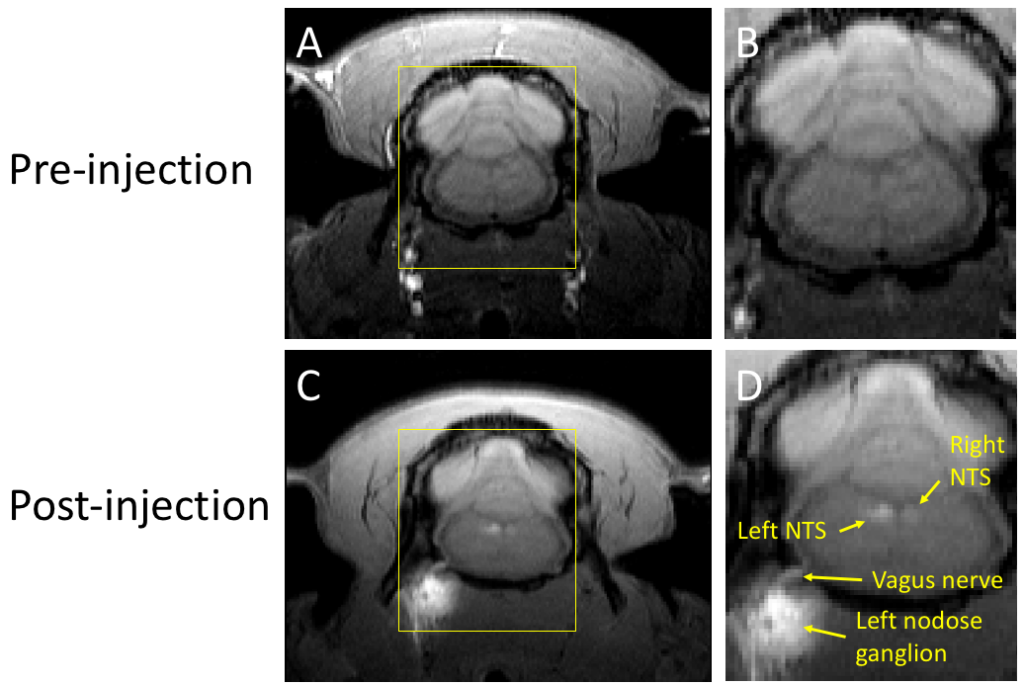
In vivo tracing of the ascending vagal projections to the brain with manganese enhanced magnetic resonance imaging
Oleson, Cao, Wang, Liu, Frontiers Neuroscience (2023)
The vagus nerve, the primary neural pathway mediating brain-body interactions, plays an essential role in transmitting bodily signals to the brain. Despite its significance, our understanding of the detailed organization and functionality of vagal afferent projections remains incomplete. In this study, we utilized manganese-enhanced magnetic resonance imaging (MEMRI) as a non-invasive and in vivo method for tracing vagal nerve projections to the brainstem and assessing their functional dependence on cervical vagus nerve stimulation (VNS). Manganese chloride solution was injected into the nodose ganglion of rats, and T1-weighted MRI scans were performed at both 12 and 24 h after the injection. Our findings reveal that vagal afferent neurons can uptake and transport manganese ions, serving as a surrogate for calcium ions, to the nucleus tractus solitarius (NTS) in the brainstem. In the absence of VNS, we observed significant contrast enhancements of around 19–24% in the NTS ipsilateral to the injection side. Application of VNS for 4 h further promoted nerve activity, leading to greater contrast enhancements of 40–43% in the NTS. These results demonstrate the potential of MEMRI for highresolution, activity-dependent tracing of vagal afferents, providing a valuable tool for the structural and functional assessment of the vagus nerve and its influence on brain activity.
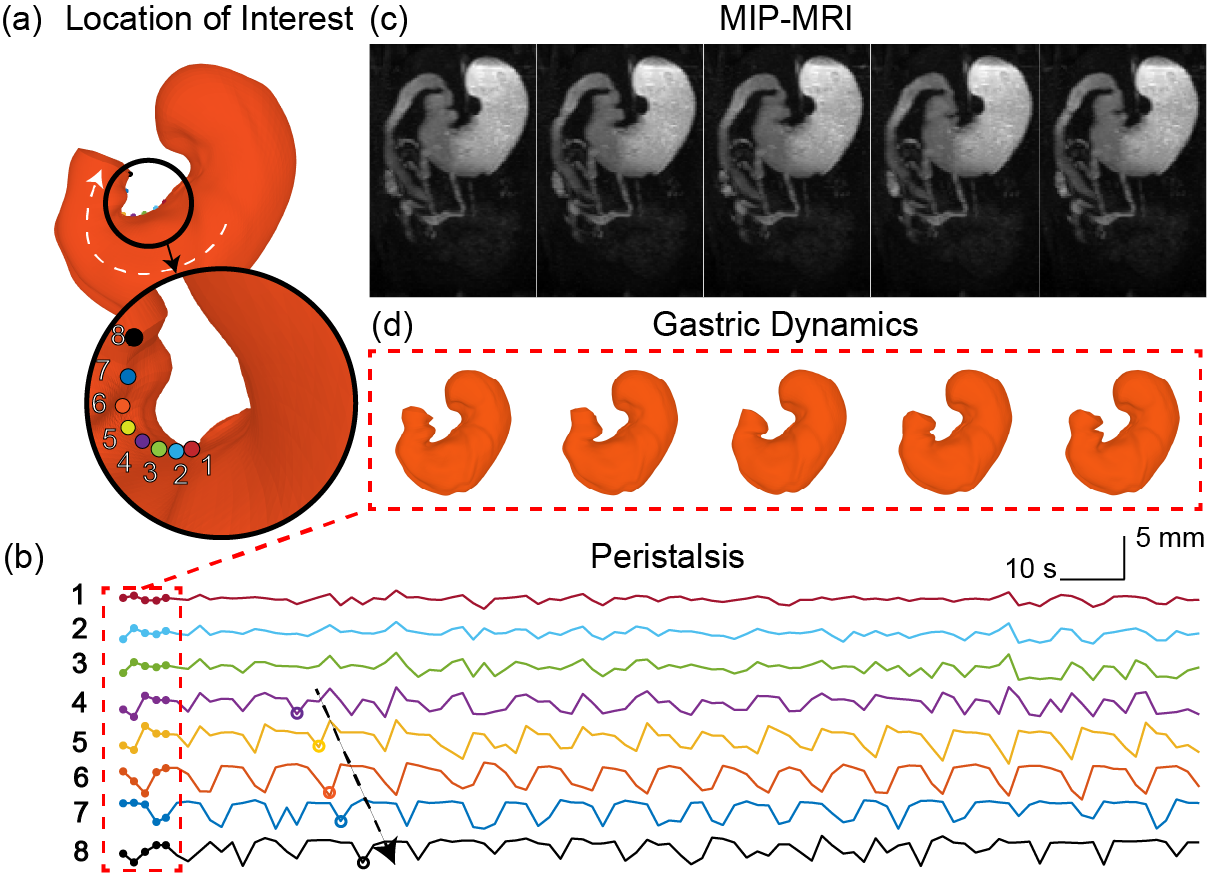
Diffeomorphic surface modeling for MRI-based characterization of gastric anatomy and motility
Wang, Cao, Han, Choi, She, Scheven, Avci, Du, Cheng, Di Natali, Furness, Liu, IEEE TBME (2023)
Gastrointestinal magnetic resonance imaging (MRI) provides rich spatiotemporal data about the volume and movement of the food inside the stomach, but does not directly report on the muscular activity of the stomach itself. Here we describe a novel approach to characterize the motility of the stomach wall that drives the volumetric changes of the gastric content. In this approach, a surface template was used as a deformable model of the stomach wall. A neural ordinary differential equation (ODE) was optimized to model a diffeomorphic flow that ascribed the deformation of the stomach wall to a continuous biomedical process. Driven by this diffeomorphic flow, the surface template of the stomach progressively changes its shape over time or between conditions, while preserving its topology and manifoldness. We tested this approach with MRI data collected from 10 Sprague Dawley rats under a lightly anesthetized condition. Our proposed approach allowed us to characterize gastric anatomy and motility with a surface coordinate system common to every individual. Anatomical and motility features could be characterized for each individual, and then compared and summarized across individuals for group-level analysis. As a result, high-resolution functional maps were generated to reveal the spatial, temporal, and spectral characteristics of muscle activity as well as the coordination of motor events across different gastric regions. The relationship between muscle thickness and gastric motility was also evaluated throughout the entire stomach wall. Such a structure-function relationship was used to delineate two distinctive functional regions of the stomach. These results demonstrate the efficacy of using GI-MRI to measure and model gastric anatomy and function. This approach described herein is expected to enable non-invasive and accurate mapping of gastric motility throughout the entire stomach for both preclinical and clinical studies.
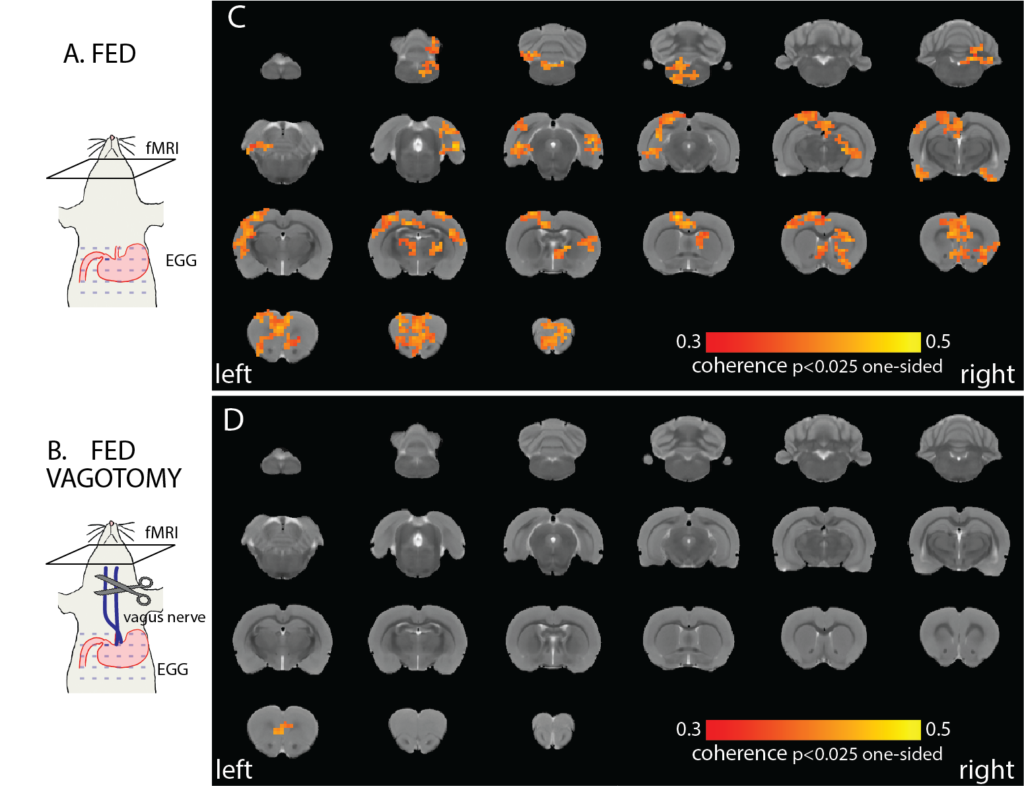
The vagus nerve mediates stomach-brain coherence in rats
Cao, Wang, Chen, Zhang, Liu, NeuroImage (2022)
Interactions between the brain and the stomach shape both cognitive and digestive functions. Recent human studies report spontaneous synchronization between brain activity and gastric slow waves in the resting state. However, this finding has not been replicated in any animal models. The neural pathways underlying this apparent stomach-brain synchrony is also unclear. Here, we performed functional magnetic resonance imaging while simultaneously recording body-surface gastric slow waves from anesthetized rats in the fasted vs. postprandial conditions and performed a bilateral cervical vagotomy to assess the role of the vagus nerve. The coherence between brain fMRI signals and gastric slow waves was found in a distributed “gastric network”, including subcortical and cortical regions in the sensory, motor, and limbic systems. The stomach-brain coherence was largely reduced by the bilateral vagotomy and was different between the fasted and fed states. These findings suggest that the vagus nerve mediates the spontaneous coherence between brain activity and gastric slow waves, which is likely a signature of real-time stomach-brain interactions. However, its functional significance remains to be established.
Gastric Neurons in the Nucleus Tractus Solitarius are Selective to the Orientation of Gastric Electrical Stimulation
Cao, Wang, Powley, Liu, Journal of Neural Engineering, 18: 056066 (2021)
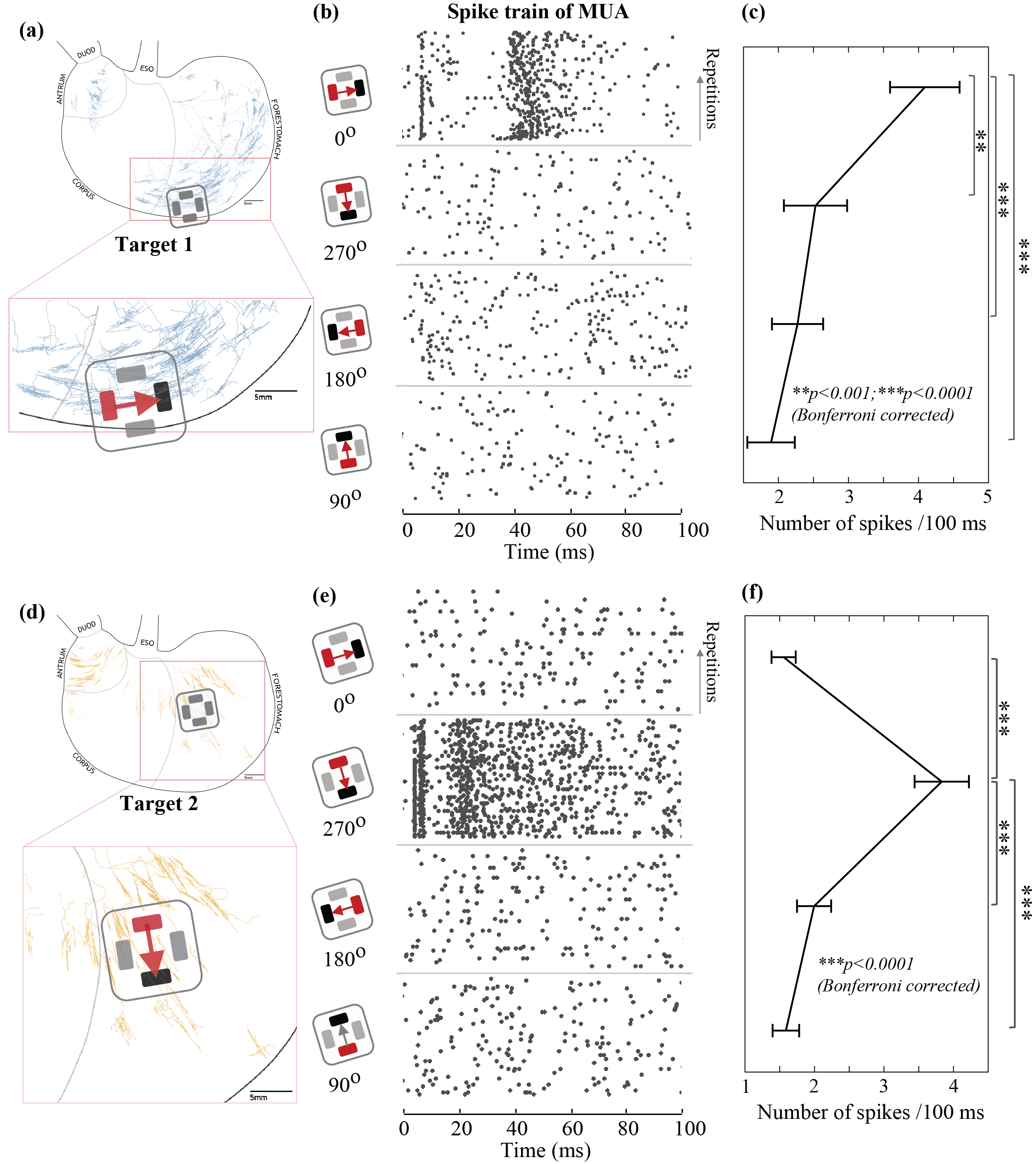
The brain monitors and regulates the stomach in part through vagal neural pathways. Although there has been increasing progress describing the structural phenotypes of vagal projections to the stomach, very little is known about the specific gastric information transmitted to and encoded by the brain. In this study, we report that gastric neurons in the nucleus tractus solitarius encode the orientation-specific activity of gastric smooth muscles. This neural code is relayed through vagal afferent neurons with their nerve terminals along circular or longitudinal muscle fibers. This finding provides a critical foundation for understanding gastric interoception. It brings up a new consideration that the orientation of gastric electrical stimulation is critical to engaging vagal afferent pathways for modulating gastric functions.
Acute effects of vagus nerve stimulation parameters on gastric motility assessed with magnetic resonance imaging
(Lu, Cao, Phillips, Powley, Liu, 2020. Neurogastroenterology and Motility, e13853)
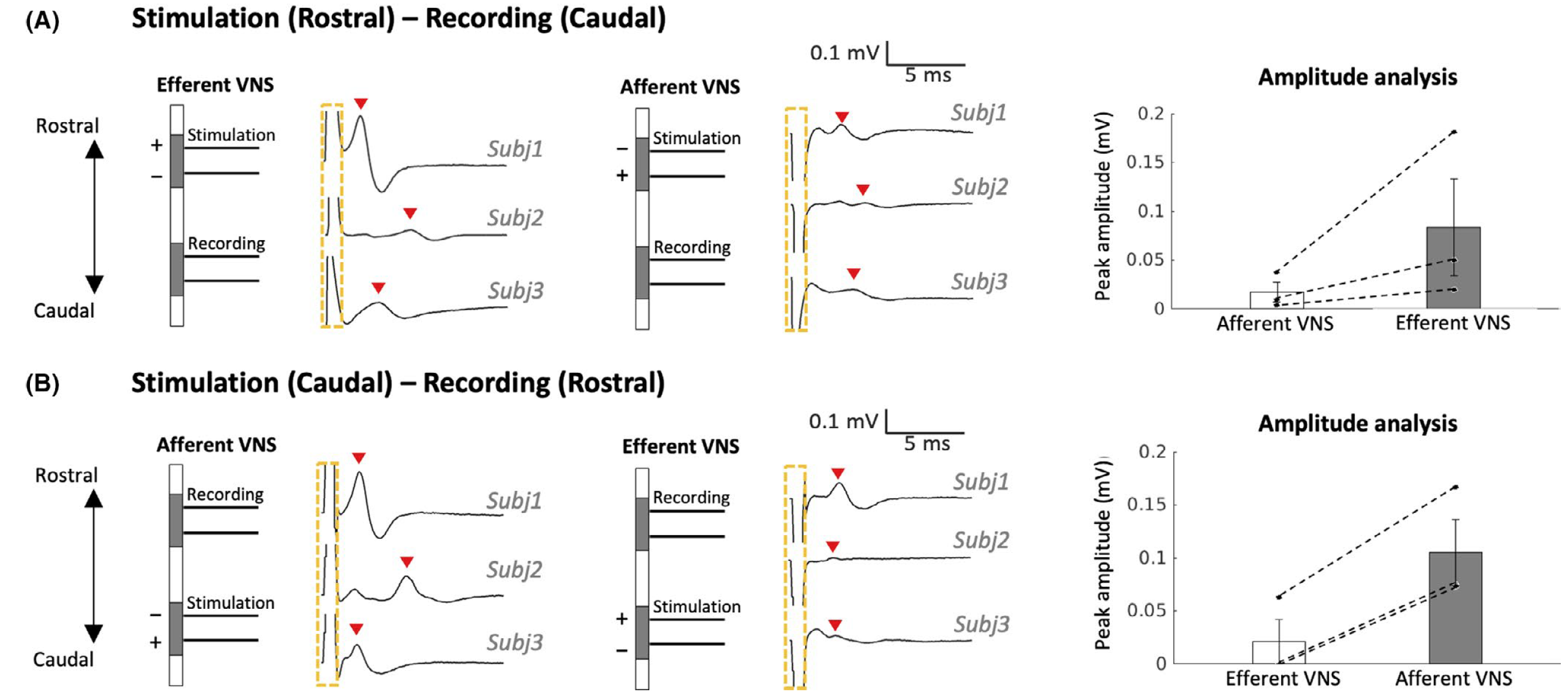
Vagus nerve stimulation (VNS) is an emerging bioelectronic therapy for regulating food intake and controlling gastric motility. However, the effects of different VNS parameters and polarity on post-prandial gastric motility remain incompletely characterized. We have found that monophasic VNS biased towards the afferent pathway is potentially more effective for facilitating occlusive contractions than that biased towards the efferent pathway.
Gastric stimulation drives fast BOLD responses
(Cao, Lu, Oleson, Phillips, Jaffey, Hendren, Powley, Liu. NeuroImage, 197: 200-211, 2019)
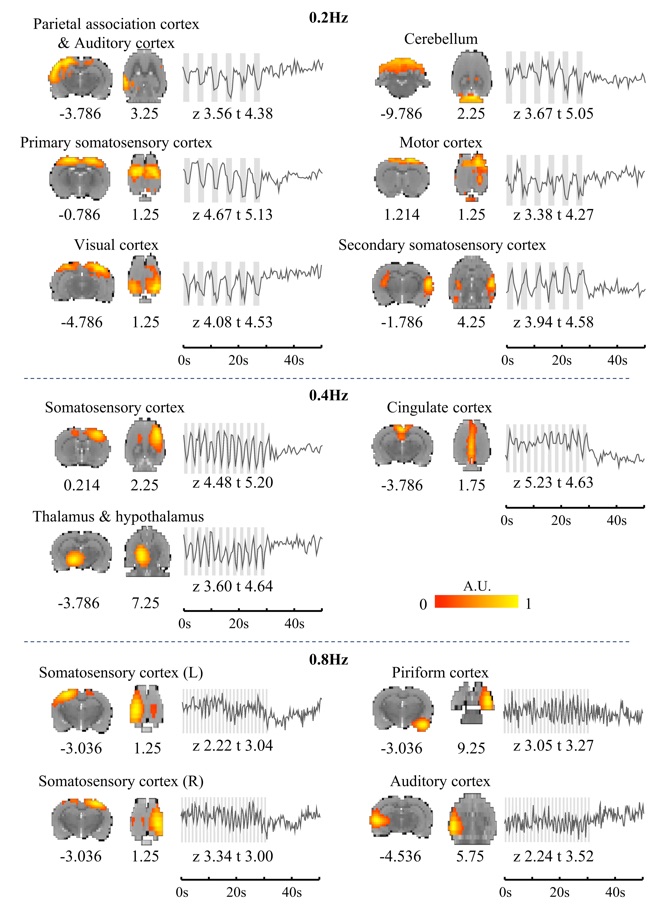
Functional magnetic resonance imaging (fMRI) is commonly thought to be too slow to capture any neural dynamics faster than 0.1 Hz. However, recent findings demonstrate the feasibility of detecting fMRI activity at higher frequencies beyond 0.2 Hz, whereas the origin, reliability, and generalizability of fast fMRI responses are under debate and remain to be confirmed through animal experiments with fMRI and invasive electrophysiology. Here, we acquired single-echo and multi-echo fMRI, as well as local field potentials, from anesthetized rat brains given gastric electrical stimulation modulated at 0.2, 0.4 and 0.8 Hz. Such gastric stimuli could drive widespread fMRI responses at corresponding frequencies from the somatosensory and cingulate cortices. Such fast fMRI responses were linearly dependent on echo times and thus indicative of blood oxygenation level dependent nature (BOLD). Local field potentials recorded during the same gastric stimuli revealed transient and phase-locked broadband neural responses, preceding the fMRI responses by as short as 0.5 sec. Taken together, these results suggest that gastric stimulation can drive widespread and rapid fMRI responses of BOLD and neural origin, lending support to the feasibility of using fMRI to detect rapid changes in neural activity up to 0.8 Hz.
Adaptive and wireless recordings of electrophysiological signals during concurrent MRI
(Mandal, Babaria, Cao, Liu. IEEE Trans. Biomed. Engr., 2018)
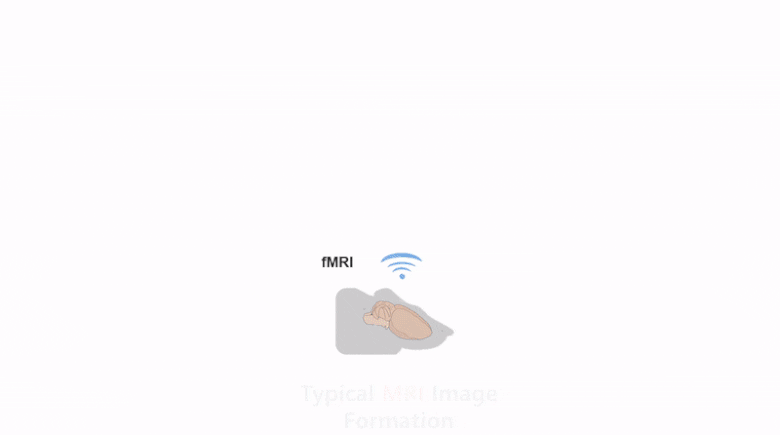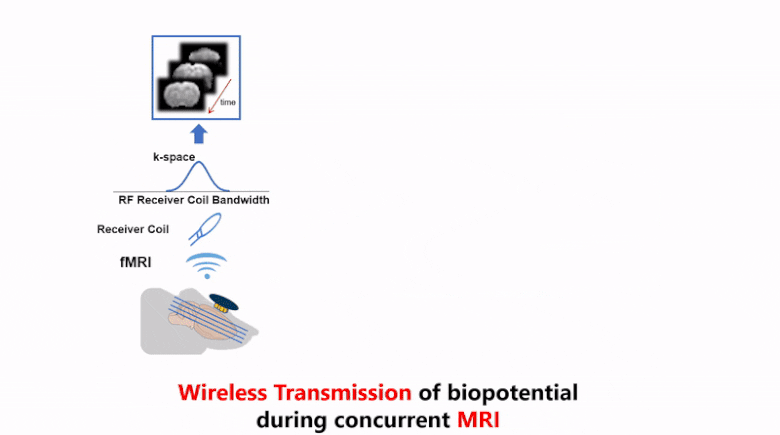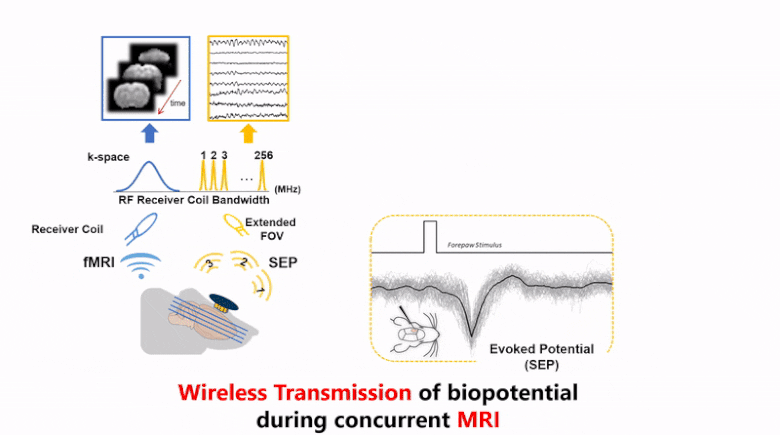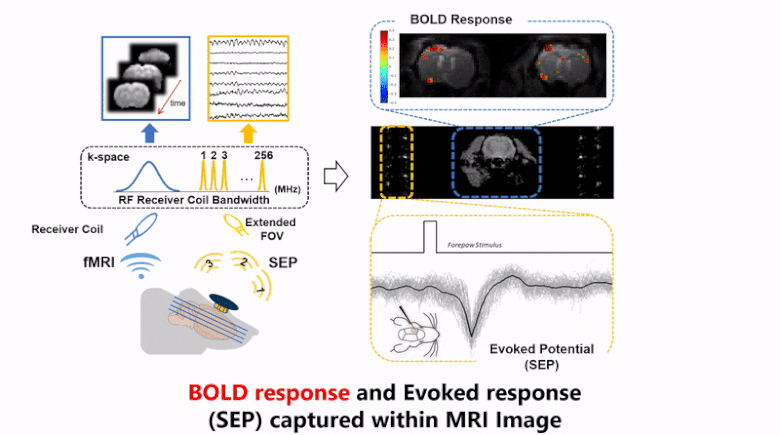
Strong electromagnetic fields during functional magnetic resonance imaging (fMRI) presents a challenging environment for any concurrent electrophysiological recording. Here, we present a miniaturized, wireless platform – “MR-Link” (Multimodal Recording Link) that provides a hardware solution for simultaneous electrophysiological and fMRI signal acquisition. The device detects changes in the electromagnetic field during fMRI and synchronizes amplification and sampling of electrophysiological signals to minimize effects of gradient and RF artifacts. It wirelessly transmits the recorded data at a frequency detectable by the MR-receiver coil. The transmitted data is readily separable from MRI in the frequency domain. To demonstrate its efficacy, we used this device to record electrocardiograms and somatosensory evoked potential without artifacts from concurrent fMRI scans, or compromising imaging quality. The compact recording device (20 mm dia., 2gms) placed within the MR-bore minimized movement artifacts and achieved microsecond-level synchronization with fMRI data. MR-Link offers an inexpensive system to eliminate the need for amplifiers with high dynamic range or sampling rate, high-power sampling, additional storage or synchronization hardware to connect with the MR-scanner. This device is expected to enable easier and a broader range of applications of simultaneous fMRI and electrophysiology in animals and humans.
Vagus nerve stimulation promotes gastric emptying
(Lu, Cao, Oleson, Phillips, Ward, Powley, Liu. Neurogastroenterology and Motility, 2018)
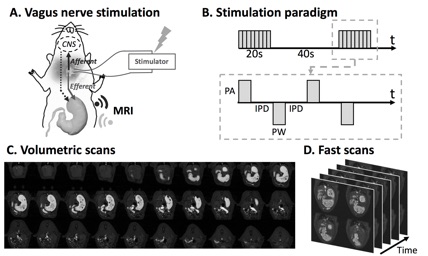
Background: Vagus nerve stimulation (VNS) is an emerging electroceutical therapy for remedying gastric disorders that are poorly managed by pharmacological treatments and/or dietary changes. Such therapy seems promising since the vagovagal neurocircuitry controlling the enteric nervous system strongly influences gastric functions. Methods: Here, the modulatory effects of left cervical VNS on gastric emptying in rats was quantified using a 1) feeding protocol in which the animal voluntarily consumed a post-fast, gadolinium-labeled meal and 2) newly developed, robust, sensitive and non-invasive imaging strategy to measure antral motility, pyloric activity and gastric emptying based on contrast-enhanced magnetic resonance imaging (MRI) and computer-assisted image processing pipelines. Key Results: VNS significantly accelerated gastric emptying (control vs. VNS: 24.9±3.5% vs. 40.7±3.9% of meal emptied per 4hrs, p<0.05). This effect resulted from a greater relaxation of the pyloric sphincter (control vs. VNS: 1.4±0.2 vs. 2.5±0.5 mm2 cross-sectional area of lumen, p<0.05), without notable changes in antral contraction amplitude (control vs. VNS: 30.6±3.0% vs. 32.5±3.0% occlusion), peristaltic velocity (control vs. VNS: 0.67±0.03 vs. 0.67±0.03 mm/s), or frequency (control vs. VNS: 6.3±0.1 vs. 6.4±0.2 cpm). The degree to which VNS relaxed the pylorus positively correlated with gastric emptying rate (r = 0.5465, p<0.01). Conclusions & Inferences: The MRI protocol employed in this study is expected to enable advanced preclinical studies to understand stomach pathophysiology and its therapeutics. Results from this study suggest an electroceutical treatment approach for gastric emptying disorders using cervical VNS to control the degree of pyloric sphincter relaxation.
Vagal nerve stimulation triggers widespread responses and alters large-scale functional connectivity in the rat brain
(Cao, Lu, Powley, Liu. 2017. PLoS ONE, 12(2): e0189518)
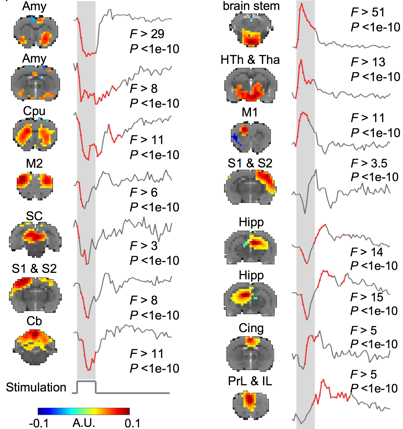
Vagus nerve stimulation (VNS) is a therapy for epilepsy and depression. However, its efficacy varies and its mechanism remains unclear. Prior studies have used functional magnetic resonance imaging (fMRI) to map brain activations with VNS in human brains, but have reported inconsistent findings. The source of inconsistency is likely attributable to the complex temporal characteristics of VNS-evoked fMRI responses that cannot be fully explained by simplified response models in the conventional model-based analysis for activation mapping. To address this issue, we acquired 7-Tesla blood oxygenation level dependent fMRI data from anesthetized Sprague-Dawley rats receiving electrical stimulation at the left cervical vagus nerve. Using spatially independent component analysis, we identified 20 functional brain networks and detected the network-wise activations with VNS in a data-driven manner. Our results showed that VNS activated 15 out of 20 brain networks, and the activated regions covered >76% of the brain volume. The time course of the evoked response was complex and distinct across regions and networks. In addition, VNS altered the strengths and patterns of correlations among brain networks relative to those in the resting state. The most notable changes in network-network interactions were related to the limbic system. Together, such profound and widespread effects of VNS may underlie its unique potential for a wide range of therapeutics to relieve central or peripheral conditions.
Contrast enhanced MRI of gastric emptying and motility in rats
(Lu, Cao, Oleson, Powley, Liu. 2017. IEEE Trans. Biomed. Engr. 64(11): 2546-2554)
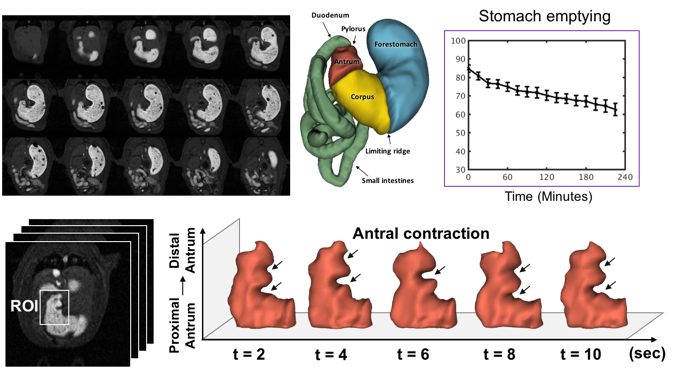
The assessment of gastric emptying and motility in humans and animals typically requires radioactive imaging or invasive measurements. Here, we developed a robust strategy to image and characterize gastric emptying and motility in rats based on contrast-enhanced magnetic resonance imaging (MRI) and computer-assisted image processing. The animals were trained to naturally consume a gadolinium-labeled dietgel while bypassing any need for oral gavage. Following this test meal, the animals were scanned under low-dose anesthesia for high-resolution T1-weighted MRI in 7-Tesla, visualizing the time-varying distribution of the meal with greatly enhanced contrast against non-GI tissues. Such contrast-enhanced images not only depicted the gastric anatomy, but also captured and quantified stomach emptying, intestinal filling, antral contraction, and intestinal absorption with fully-automated image processing. Over four post-ingestion hours, the stomach emptied by 27%, largely attributed to the emptying of the forestomach rather than the corpus and the antrum, and most notable during the first 30 minutes. Stomach emptying was accompanied by intestinal filling for the first 2 hours, while afterwards intestinal absorption was observable as cumulative contrast enhancement in the renal medulla. The antral contraction was captured as a peristaltic wave propagating from the proximal to distal antrum. The frequency, velocity, and amplitude of the antral contraction were on average 6.340.07 contractions per minute, 0.670.01 mm/s and 30.581.03%, respectively. These results demonstrate an optimized MRI-based strategy to assess gastric emptying and motility in healthy rats, paving the way for using this technique to understand GI diseases, or test new therapeutics in rat models.
Neural and physiological responses to vagus nerve stimulation in rats
(Cao, Lu, Ward, Powley, Liu. 2017. ISMRM. Magna Cum Laude Award)
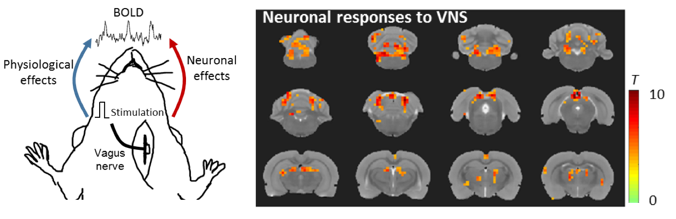
Here, we report a part of intrinsic central autonomic network in the rat brain that was modulated by the left cervical vagus nerve. VNS impacts the BOLD response in the brain in two different ways: 1) It directly triggers specific and neuronal activations at the central autonomic network and 2) it modulates cardiac and respiratory rates and in turn alters the global BOLD signal as a non-neuronal physiological effect. We demonstrate that the neuronal and non-neuronal effects are separable, offering a unique way to image and characterize the brain-activation profile of VNS applied to different locations and with variable parameters
Mapping systemic inflammation with manganese-enhanced MRI
(Lu, Cao, Marussich, Hu, Liu. 2017. ISMRM, Magna Cum Laude Award)
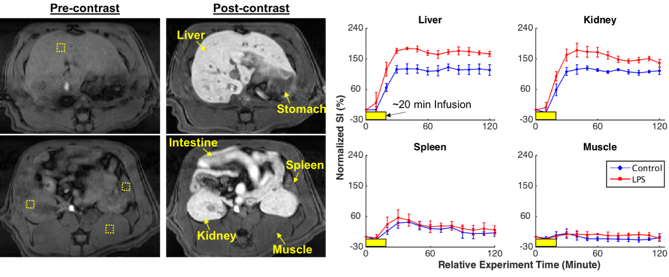
We used in vivo manganese-enhanced MRI (MEMRI) to image and assess the increase in calcium influx into immune cells so as to report the cellular responses to systemically LPS-induced inflammation throughout the abdomen. We found that: (1) The contrast enhancement was dose- and time-dependent with variation across organs. (2) An increase in Mn2+ uptake was observed in the liver and the kidney, but not in the spleen or the muscle given inflammation. (3) The inflammation-induced enhancement was dependent on the time after the initial exposure to LPS.